TCR Engineered T-Cell Therapy Status Report A Must Have Study for Academia and Pharma/Biotech Companies
The new report covers global companies like Adaptimmune, Bellicum Pharmaceuticals, Cell Medica, GlaxoSmithKline and 30+ companies.
Adoptive T-cell therapy has come of age with the recent approvals of two CAR-T therapeutics targeting CD19 (Kymriah and Yescarta) demonstrating feasibility of clinical development with impressive results and of personalized manufacturing & logistics. It remains to be seen whether these autologous CAR-Ts will live up commercial expectations, as first quarter 2018 sales of Kymriah were far below expectations (only US$ 12 mln). The final prices of US$ 475,000 (Kymriah) and US$ 373,000 mln (Yescarta) are challenging the commercial viability of autologous CAR-T therapy. Allogeneic cell therapy might be one technological solution for the pricing problem.
While CAR-Ts performed very well for hematologic malignancies, they did less for solid tumors. Furthermore, CAR-T therapy is restricted to cell surface proteins which constitute about 20-25% of all available targets on a solid tumors, leaving nearly 80% of intracellular targets untapped by CAR-T therapy or by conventional antibody therapy.
The concept of TCR T-cell therapy actually predates CAR-Ts, but suffered a series of setbacks due to toxicity caused by cross-reactivity with related and unrelated peptides prompting the development of much more sophisticated specificity screening systems. But TCR-Ts are catching up as evidenced by ten different company-related TCR-Ts entering clinical development in the last two years and further nine in preparation of clinical trials. Significant deals between TCR-T companies and major Pharma/Biotech further underline the promise of TCR-T technologies.
In this new report "TCR Engineered T-Cell Therapy 2018: an industry analysis of technologies, pipelines, stakeholders & deals" brings you up-to-date and gives you answers to key questions about
>target discovery & selection,
>technologies for generation of TCRs,
>neoantigen-specific TCRs,
>off-the-shelf TCR-Ts,
>the TCR-T pipeline,
>manufacturing strategies,
>next generation TCR-Ts,
>key players,
>competition,
>corporate strategy,
>deals and acquisitions and
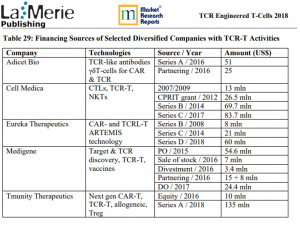
>financing.
The report can be acquired at MarketResearchReports.com at (https://www.marketresearchreports.com/la-merie-publishing/tcr-engineered-t-cell-therapy-2018-industry-analysis-technologies-pipelines ). There you will find samples pages, a detailed Table of Contents, a list of Tables and of companies discussed in the report.
Browse more reports from our Pharma and Healthcare Category: Therapeutic , Drug Pipeline
Sudeep Chakravarty
Market Research Reports Inc.
+1-302-703-9904
email us here
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.